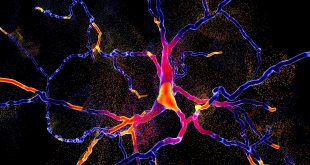
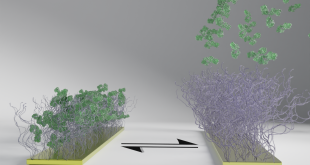
Photo Courtesy: University of British Columbia
Science has come a long way since the first blood vessels were bioprinted in 2010, successfully printing cartilage, bone, cardiac, nervous, liver, and vascular tissues. Recently, scientists at the University of British Columbia added to that list with 3D bioprinted human testicular cells, a Canadian first that offers hope for men with a severe form of infertility known as non-obstructive azoospermia (NOA).
According to UBC, the scientists, led by UBC urology assistant professor Dr. Ryan Flannigan, suggest that the technique may one day offer a solution for people living with currently untreatable forms of male infertility.
“Infertility affects 15% of couples, and male factors are a contributing cause in at least half of those cases,” said Dr. Flannigan, whose lab is based at the Vancouver Prostate Centre at Vancouver General Hospital.
“We’re 3D printing these cells into a very specific structure that mimics human anatomy, which we think is our best shot at stimulating sperm production. If successful, this could open the door to new fertility treatments for couples who currently have no other options.”
Within human testicles, sperm is produced by tiny tubes known as seminiferous tubules. In NOA, no sperm is found in ejaculate due to diminished sperm production within these structures.
While in some cases doctors can help NOA patients by performing surgery to find extremely rare sperm, Dr. Flannigan says this procedure is only successful about half the time. “Unfortunately, for the other half of these individuals, they don’t have any options because we can’t find sperm for them.”
Those are the patients Dr. Flannigan’s team is hoping to help.
For the recent study, the researchers performed a biopsy to collect stem cells from the testicles of a patient living with NOA. The cells were then grown and 3D printed onto a petri dish inside a hollow tubular structure that resembles the sperm-producing seminiferous tubules.
“3D bioprinting is a highly customizable platform that gave us control on the largest number of variables to facilitate favourable cell-cell interactions and recapitulate in vivo cytoarchitecture like we see naturally in the human body,” explains Dr. Flannigan.
The team used a 3D printer manufactured by Aspect Biosystems, which also supplied the proprietary bioink in which the cells were mixed. To determine the precise amount of biomaterial for the ink, as a starting point they relied on previously published data on cell density required for 3D bioprinting, explains Dr. Flannigan, and “then performed several series of optimization experiments of cell concentration, and printing parameters to achieve the structure achieved in this publication.”
To shape the 3D printed cells, they used a coaxial needle that consisted of a sacrificial core material (polyvinyl alcohol–PVA 6%) with cells situated in AGC10 bioink in the outer shell to achieve the tubular structure.
Twelve days after printing, the team found that the cells had survived. Not only that, they had also matured into several of the specialized cells involved in sperm production, and they were showing a significant improvement in spermatogonial stem cell maintenance — both early signs of sperm-producing capabilities. The results of the study were recently published in Fertility and Sterility Science.
“It’s a huge milestone, seeing these cells survive and begin to differentiate. There’s a long road ahead, but this makes our team very hopeful,” said Dr. Flannigan.
The team is now working to “coach” the printed cells into producing sperm. To do this, they’ll expose the cells to different nutrients and growth factors, and fine-tune the structural arrangement to facilitate cell-to-cell interaction.
If they can get the cells to produce sperm, those sperm could potentially be used to fertilize an egg by in vitro fertilization, providing a new fertility treatment option for couples.
Dr. Flannigan’s research program has also been shedding new light on the genetic and molecular mechanisms that contribute to NOA. They’ve been using various single-cell sequencing techniques to understand the gene expression and characteristics of each individual cell, then applying computational modelling of this data to better understand the root causes of the condition and to identify new treatment options. The work has been highly collaborative, involving UBC researchers across computer science, mathematics and engineering, as well as international collaborations.
“Increasingly, we’re learning that there are likely many different causes of infertility and that each case is very patient-specific,” said Dr. Flannigan. “With that in mind, we’re taking a personalized, precision medicine approach. We take cells from a patient, try to understand what abnormalities are unique to them, and then 3D print and support the cells in ways that overcome those original deficiencies.”
This research is supported by Michael Smith Health Research BC, the Canada Foundation for Innovation, Canadian Institute of Health Research, Vancouver Coastal Health Research Institute, American Society of Reproductive Medicine, Canadian Urologic Association Scholarship Foundation and the Vancouver Prostate Centre.
 BioLab Business Magazine Together, we reach farther into the Canadian Science community
BioLab Business Magazine Together, we reach farther into the Canadian Science community